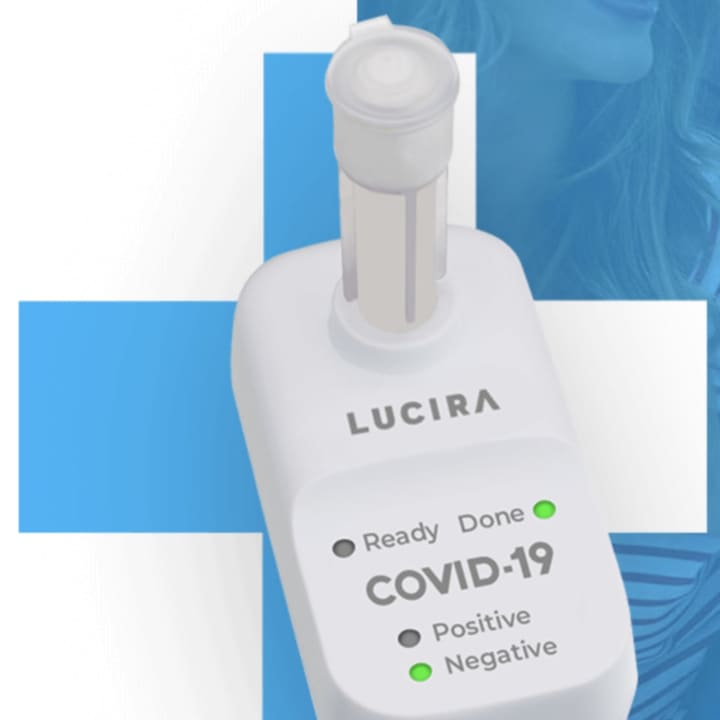
The FDA issued an emergency use authorization on Tuesday, Nov. 17 for the "Lucira COVID-19 All-In-One Test Kit," a single-use test that will detect the novel coronavirus SARS-CoV-2 that causes COVID-19.
The test works by swirling the self-collected sample swab in a vial that is then placed in the test unit. In 30 minutes or less, the results can be read directly from the test unit’s light-up display that shows whether a person is positive or negative for the SARS-CoV-2 virus.
“The FDA continues to demonstrate its unprecedented speed in response to the pandemic,” FDA Commissioner Stephen M. Hahn said. “While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home.
"This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission,” Hahn added. “Today’s action underscores the FDA’s ongoing commitment to expand access to COVID-19 testing.”
According to the FDA, the test has been authorized for at-home use in individuals over the age of 14 who suspect they have contracted COVID-19. It has also been authorized for use by healthcare professionals in minors under the age of 14.
Lucira Health said the tests were expected to initially roll out in California and Florida "in the near future." The kits will then be widely distributed across the country in spring with a price tag of $50.
According to Lucira Health, the tests have a sensitivity of about 94.1 percent and a specificity of 98 percent.
“Today’s authorization for a complete at-home test is a significant step toward FDA’s nationwide response to COVID-19,” Jeff Shuren, director of FDA’s Center for Devices and Radiological Health, stated. “A test that can be fully administered entirely outside of a lab or healthcare setting has always been a major priority for the FDA to address the pandemic.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them.”
Click here to follow Daily Voice Greenwich and receive free news updates.